Characterization of P-SA/Ins
When the GDL was added, the combination with glargine insulin was crosslinked slowly and evenly for about 30 min ~ 1 h at RT. And Macroscopic photos of the hydrogels have been proven in Fig. 1A. SEM revealed that alginate varieties a extremely crosslinked community construction after crosslinking with calcium ions, as proven in Fig. 1B. After insulin loading, the general construction of the hydrogel didn’t change considerably, indicating that insulin loading didn’t harm the construction of the hydrogel. After the grafting of PHMB was accomplished, a PHMB membrane shaped on its floor, indicating profitable PHMB loading. The profitable grafting of PHMB was verified by FI-TR spectra (Fig. 1C). Within the spectrogram of the P-SA/Ins, the attribute infrared absorption peaks of PHMB appeared at 2150 cm− 1 and 2900 cm− 1, representing the absorption peaks of the C = N bond and C-O bond, respectively. As well as, a vibrational absorption peak appeared at 1400 cm− 1, indicating amide bond formation. Determine 2D and Desk S1 present that P-SA/Ins has a swelling charge of 200%, which performs an vital position in absorbing wound tissue exudate. Glargine insulin has the power to stimulate secure and extended insulin launch for six–24 h in a impartial surroundings [26]. On this program, the acidic surroundings created by the GDL maintained the soluble state of glargine insulin. With the response of the system and cross-linking completion, the acidity was neutralized, and insulin was precipitated to realize secure and sustainable launch for not less than 96 h. The cumulative launch of insulin from SA/Ins and P-SA/Ins in PBS was roughly 689.8 ± 25.5 mIU and 664.2 ± 28.2 mIU inside 7 days (Fig. 1E).
Characterization of P-SA/Ins. A: Schematic and normal view of P-SA/Ins. B: SEM photos of every group. The scales bars: 500 μm and 100 μm. C: FI-TR spectra of gels of every group. D: The swelling ratio of every group in 37℃ PBS inside 48 h. E: The cumulative launched quantity of insulin from SA/Ins and P-SA/Ins incubated in 37℃ PBS
Biocompatibility of P-SA/Ins
AO/EB staining, CCK-8 assays and scratch assays have been subsequently carried out to guage the biocompatibility of the P-SA/Ins by observing the viability, proliferation and migration of HUVECs and HDFs. The viability of HUVECs and HDFs was assessed through an AO/EB assay. The cells have been cultured with an extraction answer of endothelial cell medium or DMEM from SA, SA/Ins or P-SA/Ins (0.05%, w/v)). Determine 2A-B exhibits that the variety of useless cells (stained orange by EB) in every group was much like that within the management group for twenty-four h, indicating that P-SA/Ins had barely toxicity. Cell proliferation underneath P-SA/Ins was detected through a CCK-8 assay. In contrast with different teams, the expansion of HDF (Fig. 2D) and HUVEC (Fig. 2E) from the SA/Ins and P-SA/Ins teams was considerably larger at 3 and 5 days. PHMB grafting had no impact on insulin-induced HDF or HUVEC development. For wound therapeutic assay, the migration charges of the HDFs and HUVECs within the SA/Ins group reached 89% and 93%, whereas these within the P-SA/Ins group reached 88% and 90%, which have been considerably larger than these within the management group (65% for HDFs and 78% for HUVECs) and the SA group (68% for HDFs and 76% for HUVECs) (Fig. 2F-I). Primarily based on these outcomes, P-SA/Ins was in a position to facilitate fibroblast and endothelial cell proliferation and migration, with barely cytotoxicity.
Biocompatibility of P-SA/Ins. A-C: Consultant photos and statistical information of Cell Toxicity assay of HDF and HUVEC cell for twenty-four h. (ns: p > 0.05, scales bars: 0.5 mm). D-E: The statistical information of cell development of HDF (D) and HUVEC (E). (** p ≤ 0.01, * p ≤ 0.05) F-G: Wound therapeutic assay and statistical information of HDF(F–G) and HUVEC (H–I). (Scale bar: 1 mm. *** p ≤ 0.001, * p ≤ 0.05)
Antibacterial capacity of P-SA/Ins
E. coli and S. aureus have been used as consultant strains of G- and G + micro organism for antibacterial capacity. Research have reported that 0.019–2% PHMB is utilized to kill bacterial [27]. The focus of PHMB grafted on the sodium alginate hydrogel was totally evaluated. An antibacterial capacity of hydrogels with PHMB concentrations on 10− 5%, 10− 4%,10− 3%, 10− 2%, 0.05%, 0.1%, 0.2%, 0.5%, 1%, 2%, and 5% w/v was carried out. A disk diffusion assay was carried out to guage antibacterial exercise by measuring the zone of inhibition (ZOI), with penicillin‒streptomycin used as a constructive management. As proven in Determine S1A-B, 10− 5%, 10− 4%,10− 3% PHMB grafted didn’t present the zone of inhibition, indicating that that they had no bactericidal impact. When the PHMB focus was larger than 0.2%, the antibacterial impact didn’t enhance with growing PHMB focus. The diameters of the ZOIs for E. coli have been roughly 12, 15, 20 mm and greater than 22 mm for 10− 2%, 0.05%, 0.1%, 0.2% PHMB-grafted. For S. aureus, roughly 10 mm, 13 mm, 15 mm and 16 mm for 10− 2%, 0.05%, 0.1%, and 0.2% PHMB-grafted, respectively. To additional optimize the focus of PHMB, the viability of HDFs cultured with extraction answer from the ten− 2%, 0.05%, 0.1%, and 0.2% P-SA/Ins was assessed by an AO/EB assay for twenty-four h. As proven in Determine S2, the variety of early apoptotic or useless cells within the 10− 2% and 0.05% teams was near that within the management group, indicating their good cytocompatibility. When the focus of PHMB was larger than 0.05% w/v, the variety of useless cells drastically elevated and reached roughly 15%, indicating an opposed impact on cell viability and cytotoxicity. Furthermore, the hydrogels immersed in PBS for 3 and 5 days nonetheless exhibited robust antibacterial results on each S. aureus and E. coli, with 12–13 mm inhibition diameters (Fig. 3A-D). Thus, 0.05% w/v PHMB was thought-about the optimum focus for P-SA/Ins.
The antibacterial impact of P-SA/Ins (0.05%, w/v) was additionally assessed through an OD worth counting assay. In contrast with different teams, the leaching tradition medium of the P-SA/Ins considerably lowered the bacterial density of S. aureus and E. coli, as proven by the bacterial optical density (Fig. 3B). A crystal violet assay for biofilm biomass indicated that P-SA/Ins was more practical in assuaging the formation of S. aureus biofilms (Fig. 3C). Visualization of the bacterial biofilms through SEM revealed a large spectrum of morphological variations (Fig. 3E). Not like the great situation and aggregation of S. aureus cells in different teams (Crimson arrow), scattered and crimpled cells (Yellow arrow) have been noticed after publicity to the leaching tradition medium of P-SA/Ins. Taken collectively, these outcomes demonstrated that P-SA/Ins successfully inhibited bacterial exercise and biofilm formation in each G- and G + micro organism.
Regulation of macrophage irritation by P-SA/Ins
Macrophages are vital inflammatory cells concerned in wound regulation [28]. To guage the impact of P-SA/Ins on the inflammatory response of macrophages within the presence of an infection, macrophages have been cocultured with S. aureus within the leaching tradition medium of every group for twenty-four h. The cell tradition supernatant was collected and analyzed through a Luminex multiplex assay to detect typical M1- and M2-related cytokines (Fig. 3F). A heatmap revealed that the P-SA/Ins group had distinguishable protein profiles from these of the opposite three teams (Fig. 3G). The degrees of Interleukin-1β (IL-1β), Interleukin-1α (IL-1α), Tumor necrosis factor-α (TNF-α) and Interleukin-6 (IL-6) within the P-SA/Ins group have been considerably decrease than these within the Ctrl group. There have been vital variations within the ranges of IL-1β, TNF-α, IL-1α and Interferon–γ (INF-γ) (p < 0.05) (Fig. 3H-Okay). As well as, the standard anti-inflammatory cytokine Interleukin-13 (IL-13) elevated (Fig. 3L). These outcomes prompt that insulin alone couldn’t successfully inhibit the inflammatory response of macrophages induced by micro organism. Suppresses bacterial exercise successfully controls the discharge of inflammatory cytokines, and the degrees of anti-inflammatory elements enhance to a sure extent.
Antibacterial and irritation regulation of P-SA/Ins in vitro. A: Schematic of antibacterial assay of P-SA/Ins. B: The statistical information of OD worth counting assay of S. aureus. (***p ≤ 0.001, n = 12). C: The statistical information of crystal violet assay for S. aureus biofilm formation (*** p ≤ 0.001, n = 9). D: The statistical information of the diameters of inhibition zones for S. aureus and E. coli. E: Consultant SEM photos confirmed S. aureus. The scales bars: 1 μm. Crimson arrow: the great situation and aggregation of S. aureus; Yellow arrow: scattered and crimpled S. aureus. F: Schematic of antibacterial and irritation regulation of P-SA/Ins (scar bar: 1 μm). G: Heatmap of inflammatory cytokines of leaching tradition medium cultured with macrophage and S. aureus. H-L: The statistical information of IL-1β, TNF-α, IL-1α, IFN-γ, IL-13 in leaching tradition medium of cultured with macrophage and S. aureus. (ns: p > 0.05, ** p ≤ 0.01, * p ≤ 0.05.)
Results of P-SA/Ins on wound therapeutic
To confirm the consequences of P-SA/Ins therapy on the therapeutic of contaminated wounds. A rat mannequin of wound an infection was established [29]. P-SA/Ins therapy was given, the therapeutic course of was recorded, and the wound samples have been collected as proven in Fig. 4A. Pictures of therapeutic wounds revealed that P-SA/Ins therapy effectively accelerated wound therapeutic at 5, 9, and 14 days (Fig. 4B-C). On Day 5, the proportion of the nonhealing wound space was 53.0 ± 3.2% within the P-SA/Ins group, and people within the Ctrl, SA, and SA/Ins teams have been 71.1 ± 2.4%, 67.2 ± 5.9%, and 60.3 ± 7.8%, respectively. On Day 9, the proportion of the nonhealing wound space within the P-SA/Ins group (9.3 ± 1.2%) was considerably lower than that within the Ctrl (32.9 ± 2.7%), SA (26.5 ± 2.5%), and SA/Ins (16 ± 2.9%) teams. On Day 14, the injuries within the P-SA/Ins group have been healed already, whereas the nonhealing wound space percentages within the Ctrl, SA, and SA/Ins teams have been 12.3 ± 7.5%, 7.2 ± 1.7%, and three.4 ± 1.5%, respectively. H&E staining revealed that the P-SA/Ins considerably accelerated the wound therapeutic charge. After 14 days of therapy, the injuries fully closed, with speedy re-epithelialization (Fig. 4D-F). The overall view of the regenerated space and the infiltration of inflammatory cells have been proven in Determine S3A-B. Furthermore, the P-SA/Ins elevated collagen deposition (Fig. 4E, G). These information recommend that P-SA/Ins promote therapeutic of contaminated wounds.
Impact of wound therapeutic of P-SA/Ins. A: The institution of rat mannequin with contaminated full thickness excision dorsal wounds. B: Pictures of therapeutic wounds of rats. C: Quantitative evaluation of proportion of wound space. (SA vs. SA/Ins., #P < 0.05; SA vs. P-SA/Ins, ***P < 0.01, **P < 0.01, *P < 0.05, n = 6). D-E: H&E (D) and Masson (E) staining of rat wounds on 14 days after therapy (Scale bar: 1 mm). F: The statistical information of the size of epidermal tongue (ns: p > 0.05, ** p ≤ 0.01, * p ≤ 0.05, n = 6). G: The statistical information of the collagen content material (ns: p > 0.05, **** p ≤ 0.0001, *** p ≤ 0.001, n = 6)
Antibacterial properties of P-SA/Ins in vivo
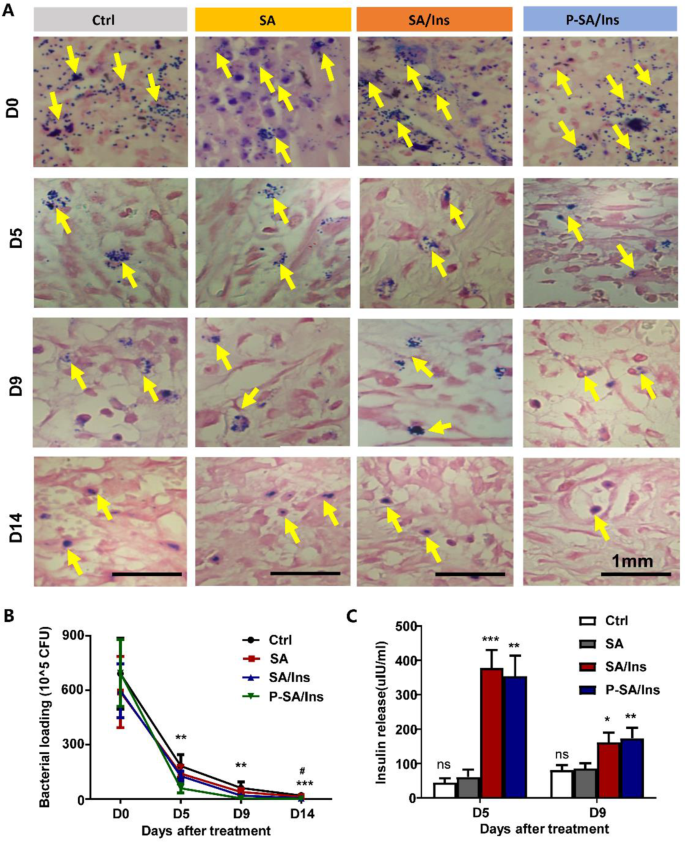
At 0, 5, 9, and 14 days after therapy, the wound tissues have been harvested and processed for bacterial evaluation. Gram staining revealed that the micro organism (Yellow arrow) within the tissues underneath P-SA/Ins therapy was considerably decrease than that within the different three teams at 0, 5, 9, and 14 days (Fig. 5A). The micro organism within the wound tissues decreased quickly in every group. Nevertheless, the P-SA/Ins therapy had considerably fewer micro organism (Fig. 5B). As well as, SA/Ins therapy additionally lowered the variety of micro organism on Day 14, which can partially attribute to immunomodulatory impact of insulin [30].
Insulin concentrations in wounds
To guage the insulin launch within the wound, the wound tissues have been harvested at 5 and 9 days. After homogenization, the insulin stage of the wound extract was detected through ELISA. Determine 5C exhibits that the insulin stage within the wound tissue from the SA/Ins (378.3 ± 51.8 mIU) and P-SA/Ins (354.3 ± 59.3 mIU) teams was considerably larger than that within the Ctrl (44.6 ± 12.4 mIU) and SA (61.2 ± 20.9 mIU) therapy teams. An identical pattern was additionally noticed on Day 9 (SA/Ins: 161.8 ± 28.4 mIU; P-SA/Ins: 173.5 ± 30.4 mIU; Ctrl: 80.9 ± 14.2 mIU; SA: 85.9 ± 15.1 mIU), suggesting that insulin was launched successfully into the injuries.
Antibacterial impact of P-SA/Ins in wound therapeutic. A: The consultant photos of Gram stain to detect S. aureus (Yellow arrow) in wound tissue on 14 days after therapy (Scale bar: 1 mm). B: The quantity of S. aureus in wound tissue after therapy for 0, 5, 9, and 14 days. Ctrl vs. SA/Ins, # P < 0.05. Ctrl vs. P-SA/Ins, ***P < 0.01, **P < 0.01; n = 6). C: The insulin focus in wound. (***P < 0.01, **P < 0.01, *P < 0.05, n = 6)
Regulation of contaminated wound irritation by P-SA/Ins
To look at the inflammatory state within the wound, typical inflammatory elements and M1/M2 markers have been detected within the wound. Multicolor fluorescence was used to find out the proportion of iNOS-marked M1 macrophages and Arg-1-marked M2 macrophages within the wound on Day 9 after therapy (Fig. 6A). In contrast with these within the Ctrl (39.67(:pm:)10.08%) and SA (26.33(:pm:)4.19%) teams, the numbers of macrophages in wounds within the SA/Ins (20.33(:pm:)0.94%) have been decreased. Nevertheless, the proportion of macrophages was additional lowered (14.67(:pm:)3.09%), with Arg-1-marked macrophages (55(:pm:)0.09%) being the predominant phenotype within the P-SA/Ins group (Determine S4). Collectively, these observations point out that P-SA/Ins decreased macrophage infiltration and promotes the M2 phenotype in wounds. Moreover, excessive quantities of the inflammatory and anti inflammatory elements IL-13, IL-10, IL-1β, and TNF-α have been noticed within the Ctrl, SA, SA/Ins teams at Days 5 and 9 after therapy. P-SA/Ins therapy considerably elevated the extent of IL-13, blunted the degrees of Interleukin-10 (IL-10) and the inflammatory elements IL-1β and TNF-α, indicating that P-SA/Ins markedly alleviated irritation within the wounds (Fig. 6B-E).
Optimized therapeutic of contaminated wounds by P-SA/Ins
Since contaminated wounds are susceptible to blisters and repeated ulcers as a result of poor epidermal and dermal connections, we additional explored the consequences of the P-SA/Ins on the therapeutic high quality of contaminated wounds on Day 14. In contrast with the opposite 3 teams, the dermis (Black sprint line) was intently related to the dermis with intensive pores and skin nails (Crimson arrow), as proven by Masson’s staining underneath P-SA/Ins therapy (Fig. 7A, C). Immunofluorescence of keratin 14 (White arrow) prompt wonderful continuity of the basement membrane (White dashed line) in Fig. 7B. Tight junction ZO-1 (White arrow) staining revealed compact junctions between keratinocytes, indicating higher integrity and stability of the pores and skin barrier (Fig. 7C-E). Thus, we decided that the P-SA/Ins optimized the therapeutic of contaminated wounds through speedy re-epithelialization and strengthen the pores and skin barrier and basement membrane.
Optimized therapeutic of contaminated wounds by P-SA/Ins. A: The pores and skin nails in zoom-in of Masson staining. Scale bar: 250 μm. Black dashed line: the dermis. Crimson arrow: basement membrane and pores and skin nails. B: Immunofluorescent staining confirmed K14, ZO-1in rat wounds on 14 days after therapy. Scale bar: 50 μm. White dashed line: basement membrane. White arrow: Expression and distribution of K14, ZO-1 C: The statistical information of pores and skin nails in rat wounds. (ns: p > 0.05, **P < 0.01, n = 5) D: The statistical information of K14 constructive charge cell in rat wounds. (ns: p > 0.05, **P < 0.01, n = 5). E: The statistical information of ZO-1 constructive charge cell in rat wounds. (ns: p > 0.05, *P < 0.05, n = 5)