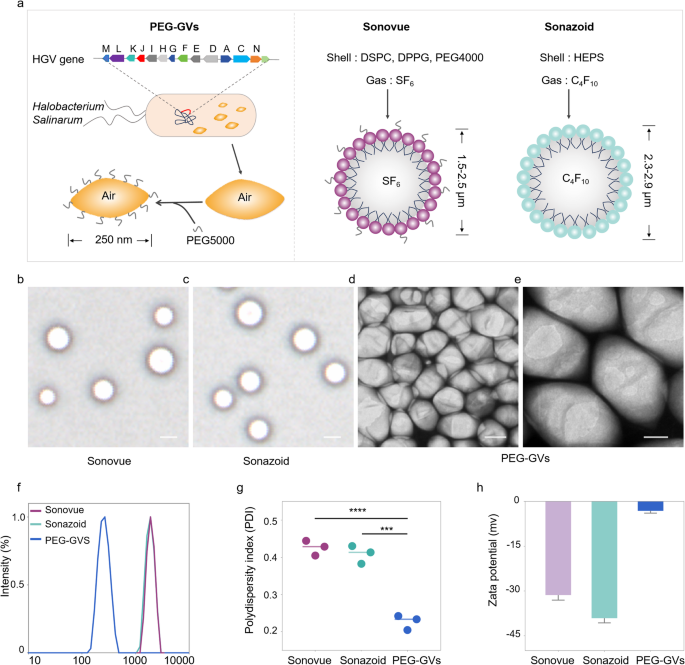
Preparation and characterization of PEG-GVs
In contrast to Sonovue and Sonazoid produced via chemical synthesis with lipid elements and inert gases like sulfurhexafluoride (SF6) or perfluorobutane (C4F10), GVs are synthesized through a organic synthesis route. Typically, GVs are encoded by gvp gene clusters within the genome and assembled into gas-filled protein shells together with the expansion of microorganisms. Firstly, we extracted GVs from Halobacterium Salinarum via lysis technique as reported beforehand [24,25,26]. To cut back their immunogenicity to physique, PEG5000 was subsequently conjugated onto the floor of GVs via amidation response within the presence of EDC and NHS, ensuing within the formation of PEG-GVs (Fig. 2a, left panel). The construction and composition of PEG-GVs additionally diverge considerably from Sonovue and Sonazoid. PEG-GVs characteristic gvp protein shells and air core. In distinction, Sonovue consists of DSPC/DPPG shells encapsulating an SF6 core, and Sonazoid predominantly consists of hydrogenated egg phosphatidyl serine (HEPS) shells with a C4F10 core (Fig. 2a, proper panel) [27]. This structural disparity can also be mirrored of their morphological look. Below the microscope, Sonovue and Sonazoid each exhibit a spherical form, whereas PEG-GVs show an ovary form below the scanning electron microscope (SEM) (Fig. 2b-2e). The hydrodynamic measurement of the UCAs was additionally measured through dynamic gentle scattering (DLS). Notably, GVs and PEG-GVs are considerably smaller, with a imply hydrodynamic diameter of 213.60 ± 1.71 nm, 252.90 ± 3.08 nm, respectively (Determine S1), versus Sonovue and Sonazoid which have particle sizes within the micrometer vary: 2.18 ± 0.78 μm and a pair of.36 ± 0.95 μm, respectively (Fig. 2f). The polydispersity index (PDI) additional differentiates these brokers. The worth of this index for PEG-GVs is 0.23 ± 0.02, which is considerably decrease than that of Sonovue 0.43 ± 0.02 and Sonazoid 0.41 ± 0.02 (Fig. 2g). In our in vitro commentary experiments, we additionally discovered that in comparison with Sonovue and Sonazoid, PEG-GVs exhibit superior dispersion traits and long-term stability (Determine S2). The zeta potentials of PEG-GVs, Sonovue, and Sonazoid are measured at -3.17 ± 0.78 mV, -31.38 ± 1.69 mV, and − 39.16 ± 1.55 mV, respectively (Fig. 2h). Thus, our outcomes show that PEG-GVs have uniform nanoscale particle measurement and excellent stability.
Preparation and characterization of PEG-GVs. (a) Composition and construction diagram of biosynthetic PEG-GVs, Sonovue and Sonazoid. b–c, the morphologies of Sonovue (b) and Sonazoid (c) noticed below microscope. Scale bar: 2.5 μm. d–e, SEM photographs of PEG-GVs. Scale bar: 100 nm (d), 50 nm (e). f–h, Particle measurement distribution (f), PDI (g) and Zeta potential (h) of PEG-GVs, Sonovue and Sonazoid. *** p < 0.001, **** p < 0.0001
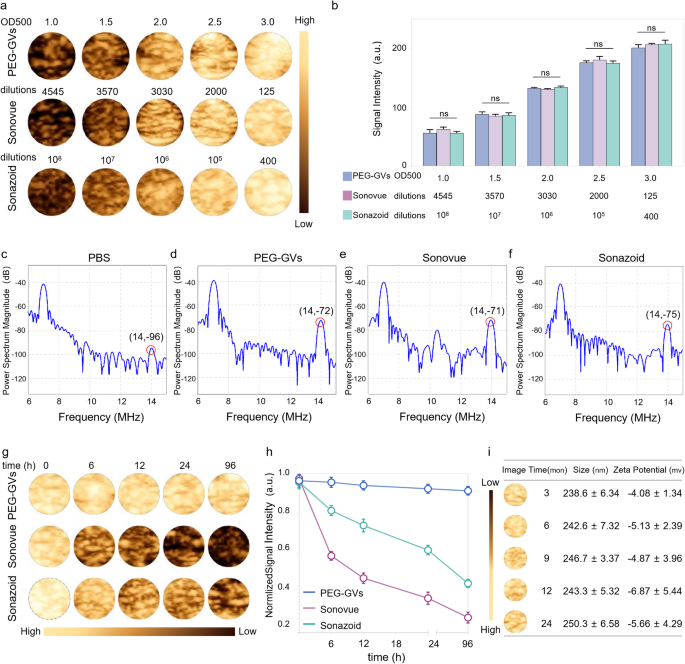
Acoustic traits and in vitro imaging of PEG-GVs
To facilitate comparability of PEG-GVs with Sonovue and Sonazoid in subsequent experiments, we first optimized the concentrations of PEG-GVs to realize a comparable imaging depth to Sonovue and Sonazoid. As depicted in Fig. 3a-3b, we will see that the CEUS imaging intensities of PEG-GVs at OD500 1.0, 1.5, 2.0, 2.5 and three.0 had been comparable with Sonovue at 4545-, 3570-, 3030-, 2000-, 125-fold dilutions and Sonazoid at 108-, 107-, 106-, 105-, 400-fold dilutions, respectively. Typically, nonlinear imaging modes depend upon harmonic alerts from microbubble or nanobubble oscillations excited by ultrasound waves, bettering distinction specificity of CEUS. Particularly, the second harmonic alerts are essential for CEUS imaging. Due to this fact, we in contrast the harmonic sign traits of PEG-GVs, Sonovue and Sonazoid. Transmitting at 7 MHz, we noticed substantial second-harmonic alerts in PEG-GVs at 14 MHz in comparison with PBS management. As anticipated, Sonovue and Sonazoid produced substantial second-harmonic alerts at 14 MHz totally different from Sonazoid and PEG-GVs, Sonovue additionally produced ultraharmonic alerts (3/2 f0) at 10.5 MHz (Fig. 3e). This phenomenon primarily arises from versatile and nonlinear deformable phospholipid shells and was additionally noticed by different group [28]. Quantitative evaluation confirmed the second harmonic alerts of Sonovue, Sonazoid and PEG-GVs had 25.85 ± 1.70 dB, 22.57 ± 3.03 dB, and 24.00 ± 1.29 dB larger energy spectrum magnitude than the PBS resolution on the transmitted frequency (Determine S3). Notably, PEG-GVs produced comparable nonlinear second-harmonic sign intensities, related with the business Sonovue, Sonazoid. To check the imaging stability of those UCAs, PEG-GVs (OD500 3.0) and Sonovue and Sonazoid (at corresponding concentrations with comparable CEUS imaging intensities) had been stored at 4 °C and imaged at totally different time intervals (Fig. 3g-h). The outcomes confirmed that the distinction imaging depth of Sonovue quickly declined to beneath 50% in a number of hours and beneath 30% after 96 h, whereas that of Sonazoid declined extra slowly but additionally dropped beneath 50% at 96 h. In stark distinction, PEG-GVs confirmed no important sign lower through the 96-h commentary interval (Fig. 3h). Moreover, it was additionally noticed that Sonovue and Sonazoid considerably vanished over time, whereas the PEG-GVs largely retained their authentic sign depth (Fig. 3g), indicating the wonderful imaging stability of PEG-GVs relative to business Sonovue and Sonazoid. To additional assess the long-term stability of PEG-GVs, we prolonged the commentary interval to 24 months and detected GVs’ imaging efficiency, particle measurement and zeta potential. We discovered that PEG-GVs nonetheless remained steady in imaging efficiency, particle measurement and zeta potential all through 24-month commentary interval (Fig. 3i). These outcomes point out that PEG-GVs stored wonderful CEUS imaging efficiency and sturdy stability, facilitating their biomedical utility and long-term preservation.
Acoustic traits and in vitro imaging of PEG-GVs. a-b, In vitro nonlinear distinction imaging (a) and quantification (b) of PEG-GVs, Sonovue and Sonazoid on the totally different concentrations. c-f, In vitro nonlinear sign spectra of PBS resolution (c), PEG-GVs (d), Sonovue (e) and Sonazoid (f). g-h, The distinction photographs (g) and quantification (h) of the three UCAs stored at 4°C for various time from 0 h to 96 h. i, In vitro nonlinear distinction imaging, particle measurement, and zeta potential of PEG-GVs stored at 4°C for 3, 6, 9, 12, 24 months.
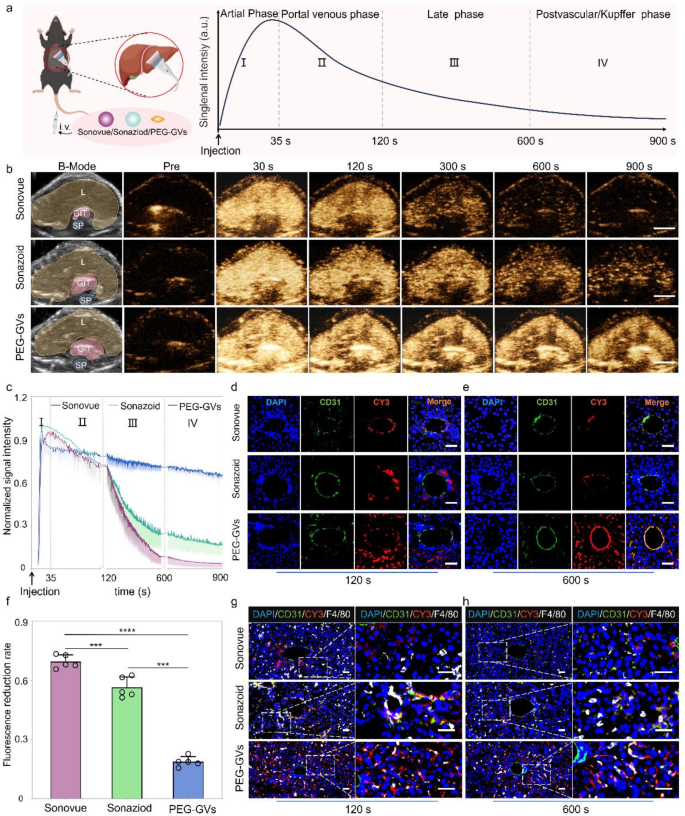
In vivo CEUS imaging of PEG-GVs in regular liver tissue
Based mostly on the excellent stability and imaging efficiency of PEG-GVs in vitro, we additional in contrast the liver distinction imaging efficiency of PEG-GVs with Sonovue and Sonazoid in wholesome C57BL/6J mice. The experimental course of and phases of ultrasound distinction agent infusion are depicted in Fig. 4a, displaying the everyday distinction echo sign enhancement modes within the arterial section, portal venous section, late section and postvascular section (additionally known as Kupffer section for Sonazoid). To cut back particular person variations, we intravenously administered these UCAs in the identical mice at 30-min intervals, adopted by a brief high-power burst earlier than subsequent injection. As proven in Fig. 4b, the echo alerts of liver acquired with Sonovue had been enhanced within the arterial section (30 s), subsided within the portal venous section (120 s), and weakened quickly after 300 s. Sonazoid has a chronic imaging time in Kupffer section and gives extra particulars for liver illness analysis. Our outcomes present that the liver echo sign of Sonazoid was considerably enhanced within the arterial section (30 s), barely diminished within the delayed section (300 s), considerably decreased within the Kupffer section (600 s), and changing into weak after 900 s. Curiously, the liver echo with PEG-GVs remained steady throughout all phases, a lot stronger than Sonovue and Sonazoid, particularly within the late and Kupffer phases (Fig. 4b). Quantitative information additional revealed that the imaging depth of Sonovue and Sonazoid declined quickly subsequent to the portal section (120 s), whereas PEG-GVs remained at a excessive degree, with a decline charge of lower than 20% in 600 s (Fig. 4f) and nonetheless lower than 30% till 900 s (Fig. 4c). The normalized AUC (Space below curve) 900 s of PEG-GVs was better than that of Sonazoid and Sonovue, being 2.72- instances of Sonazoid and 1.82 instances of Sonovue, respectively (Determine S4). Clearly, this extended imaging period presents longer time for evaluation, thereby resulting in extra exact analysis and environment friendly interventional operation for docs.
CEUS imaging efficiency and mechanisms of PEG-GVs in regular liver. a, Diagram of regular liver CEUS and its 4 phases when imaging of liver in vivo, scale bar: 5 mm. b, The CEUS photographs of various phases in the identical mice after systemic administration of Sonovue, Sonazoid and PEG-GVs at 30 min intervals. The “L”, “GIT”, “SP” represents liver, abdomen and intestines, and backbone on the B-mode photographs, respectively. c, The normalized sign depth curves of CEUS photographs in Fig. 4b. d–f, Immunofluorescence photographs of vessels (stained with anti-CD31) and CY3-labeled UCAs at 120 s (d) and 600 s (e) after systemic administration. f, The quantification of fluorescence discount charge in Fig. 4d. Scale bar: 40 μm. *** p < 0.001, **** p < 0.0001.(n = 5), g–h, Immunofluorescence photographs of liver tissues at 120 s (g) and 600 s (h) after systemic administration. Kupffer cells had been stained with anti-F4/80 and UCAs had been stained by CY3 dye. Scale bar: 40 μm.
To additional elucidate the mechanisms underlying extended imaging period, we carried out immunofluorescence staining utilizing CY3-labeled PEG-GVs and DIO-labeled Sonazoid and Sonovue. Immunofluorescence staining of liver sections revealed a substantial quantity of PEG-GVs penetrated past blood vessels and achieved liver cells. In distinction, scarce Sonazoid and Sonovue might infiltrate blood vessels and settled round blood vessels. Notably, we witnessed the progressive adhesion of PEG-GVs to the vascular partitions after systemic administration. Bubble adhesion was nonetheless observable at 120 s and annular adhesion to your entire blood vessel wall at 600 s after injection (Fig. 4d, 4e, Determine S5). This PEG-GVs’ adherence to the blood vessel partitions could possibly be noticed for as much as 1 h (Determine S6), which could partly clarify their extended imaging period of liver. Contemplating Sonazoid’s distinct benefit within the Kupffer section as UCAs, we additional investigated Kupffer cell uptake of various UCAs in liver tissue. Our findings revealed that Sonazoid and PEG-GVs, however not Sonovue, had been conspicuously internalized by Kupffer cells at 120 s and 600 s. Furthermore, the uptake of PEG-GVs elevated over time (Fig. 4g, 4h). Collectively, our information indicated PEG-GVs possesses excellent CEUS functionality within the liver, which can attribute to their adhesion and penetration past blood vessels and phagocytosis by Kupffer cells.
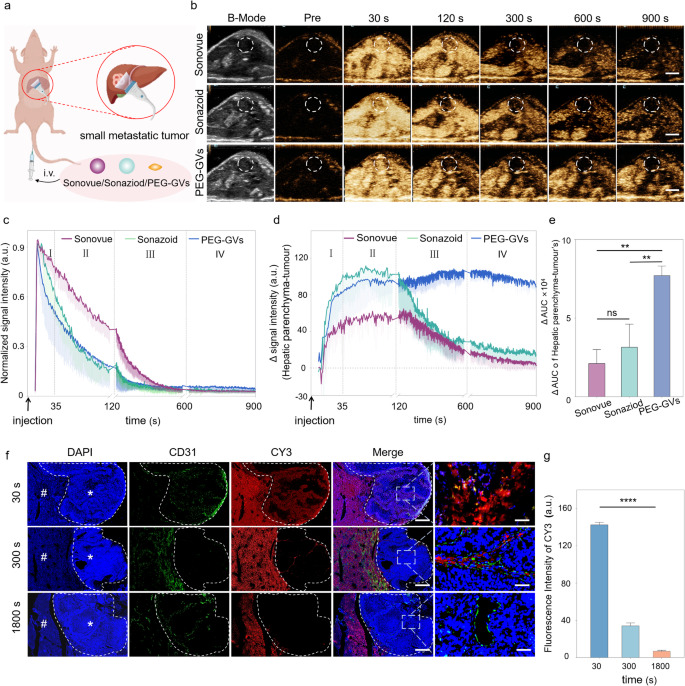
In vivo CEUS imaging of PEG-GVs in small liver tumor
The Kupffer section in liver CEUS has been demonstrated to reinforce the detection charge and specificity of small liver tumor lesions [3, 29]. In gentle of the discovering that Kupffer cells can phagocytose PEG-GVs, we hypothesized that they may play a big function within the analysis of liver tumors. To check it, we employed a murine mannequin of liver metastatic tumors to discover the potential worth of PEG-GVs in detecting minuscule hepatic lesions (Fig. 5a). After the systemic administration, all of the three UCAs exhibited speedy perfusion and speedy regression within the tumor (Fig. 5b-5c). Moreover, we noticed a sustained excessive enhancement of PEG-GVs within the regular liver tissue, attributing to their vascular adhesion and Kupffer cell uptake as described above. Curiously, PEG-GVs didn’t exhibit a sustained enhancement in liver tumor tissue because of EPR results however regressed quickly. This results in a persistently decrease imaging depth of tumors relative to peripheral liver tissue, particularly within the late section and Kupffer section (Fig. 5d). Quantitative evaluation of tumor-subtracted hepatic parenchyma AUC revealed PEG-GVs had 3.62-fold and a pair of.44-fold larger than Sonovue and Sonazoid, respectively (Fig. 5e). The immunofluorescence outcomes additional confirmed that PEG-GVs didn’t accumulate within the tumor space over time however declined quickly as an alternative, whereas it scarcely decreased within the surrounding regular liver tissue. This could be attributed to the truth that PEG-GVs don’t adhere to the vascular endothelium of tumor tissue, thereby failing to stay within the blood vessels for an prolonged interval (Fig. 5f, Determine S7). Quantitative information additionally demonstrated that PEG-GVs within the tumor decreased to solely 24.3% of peak depth after 300 s of injection and fewer than 10% after 1800 s (Fig. 5g).
CEUS imaging efficiency and mechanism of PEG-GVs in liver tumor. a, Diagram of CEUS of three UCAs in small liver metastatic tumors. b, CEUS photographs of Sonovue, Sonazoid and PEG-GVs at totally different time factors after systemic administration,scale bar: 5mm. c, The normalized sign depth curves of tumors in Figure5b. d-e, CEUS sign depth of peripheral liver tissue (d) and the quantification of AUC distinction between the encircling liver tissue and tumor at 900 s (e). f-g, Blood vessels immunofluorescence staining by anti-CD 31 (f) and quantification (g) of liver metastatic tumors at 30 s, 300 s, 1800 s after CY3-labeled PEG-GVs administration. # and * symbolize regular liver and tumor space, respectively. Scale bar: 1000 µm. **** p < 0.0001.
PEG-GVs are nanoscale with roughly 250 nm particle measurement whereas the endothelial area in neovascularized tumors is 380–780 nm, making them simply move via the tumor vessel through the EPR impact [30, 31]. The imaging efficiency of PEG-GVs might assist visualize the EPR course of of those nanobubbles by ultrasound in an actual time method. In the meantime, the speedy regression of PEG-GVs from liver tumor additionally challenges the normal idea that the EPR impact inflicting from tumor leaky vessel doesn’t essentially lengthen the retention time of nanoparticles in a tumor, no less than for nanobubbles corresponding to PEG-GVs. This distinctive phenomenon additionally renders it possible for PEG-GVs to precisely outline tumor boundaries, thereby facilitating early analysis. As for the mechanisms, the potential causes could be attributed to the excessive permeability of tumor blood vessels, which endows these nanobubbles with larger penetrating functionality into the tumor, however in the meantime it could additionally facilitate their simpler washout from the tumor in comparison with the conventional liver tissue. The structural defect of glycocalyx on the floor of tumor blood vessels can also contribute to the quick speedy regression of PEG-GVs from liver tumor [32, 33]. These outcomes indicate that PEG-GVs, based mostly on their long-time retention within the regular liver and speedy regression within the tumor, can function an excellent distinction agent within the analysis of early liver tumors and assist to supply a definite show of tumor boundaries. Furthermore, we discovered that the improved distinction sign distinction between the tumor and surrounding liver tissue can last as long as 15 min (900s), which could possibly be useful for imaging guided procedures corresponding to minimally invasive remedies of liver tumors.
Function of PEG-GVs in guiding liver tumor RFA in vivo
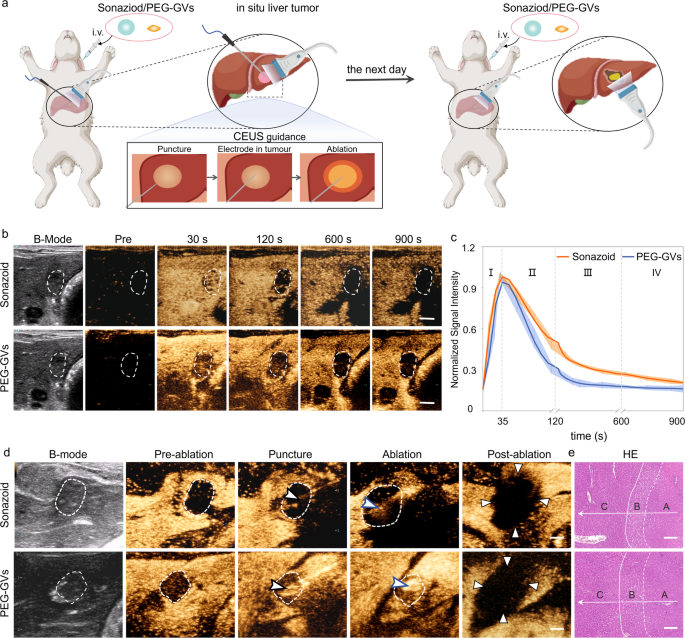
Given the nice potential of PEG-GVs in ultrasound imaging of the liver and liver tumors, we additional investigated their function in guiding radiofrequency ablation (RFA) in an in situ rabbit liver tumor mannequin (Determine S8a). As is well-known, the distinct show of tumor boundaries is crucial for figuring out tumor ablation extent. Nonetheless, the tumor boundaries normally can’t be clearly recognized on grayscale ultrasound. In distinction, CEUS can exactly find the lesion, information the puncture, and promptly consider the therapeutic impact throughout ablation. Contemplating that Sonazoid has been extensively utilized in guiding RFA ablation, we performed a comparability between Sonazoid and PEG-GVs for guiding ablation of liver tumor [34, 35]. Sonazoid or PEG-GVs had been administered intravenously to rabbits with orthotopic VX2 tumors after which carried out the ultrasound-guided RFA, adopted by a second administration subsequent day (Fig. 6a). As illustrated in Fig. 6b, each PEG-GVs and Sonazoid exhibited a broader spectrum of tumors in comparison with their two-dimensional B-mode photographs. Notably, the tumor vary displayed by PEG-GVs was even bigger and remained sturdy over time. The normalized sign depth curves indicated that each PEG-GVs and Sonazoid exhibited speedy perfusion and regression in early liver tumors (Fig. 6c). Nonetheless, PEG-GVs retained within the surrounding liver tissue for an extended interval, producing extra distinct tumor boundaries. The tumor boundaries displayed by PEG-GVs make it extra acceptable for guiding exact tumor ablation, particularly for guiding punctures and observing the ablation vary by ultrasound in a real-time method, as depicted in Fig. 6d. HE staining clearly revealed regular liver tissue, peritumoral edematous liver tissue, and coagulated necrotic liver tumor tissue after RFA, indicating that full ablation was achieved in each teams (Fig. 6e and Determine S8b). These outcomes recommend that PEG-GVs performs an analogous function to Sonazoid in guiding RFA of liver tumors.
Function of PEG-GVs in guiding RFA of liver tumor. a, Diagram of and CEUS-guided RFA therapy after administration of Sonazoid or PEG-GVs in rabbits in situ liver tumor. b–c, CEUS photographs in numerous phases of rabbits in situ liver tumor (b) and normalized sign depth curves (c), (n = 5). Scale bar: 5 mm. d, B-mode ultrasound photographs of preoperative tumor, revealing the unclear tumor boundary (white dotted circles). The tumor boundary could possibly be seen in CEUS picture until the portal venous section (white dotted circles). With the CEUS steering, monitoring puncture, radiofrequency electrodes (black arrowheads) had been simply inserted into the index tumor. The electrode needle was turned on and ablation had been carried out (blue arrowheads). The CEUS was repeated after RFA ablation. Triangle factors to the world of non-enhancement within the early arterial section, indicating a necrotic tumor instantly after RFA. (n = 3). Scale bar: 5 mm. e, HE staining of the ablated tissue. A, B, C stand for coagulated necrotic liver tumor after RFA, peritumoral edematous liver tissue and regular liver tissue, respectively (n = 3). Scale bar: 200 μm.
CEUS imaging and biosafety of PEG-GVs in macaque
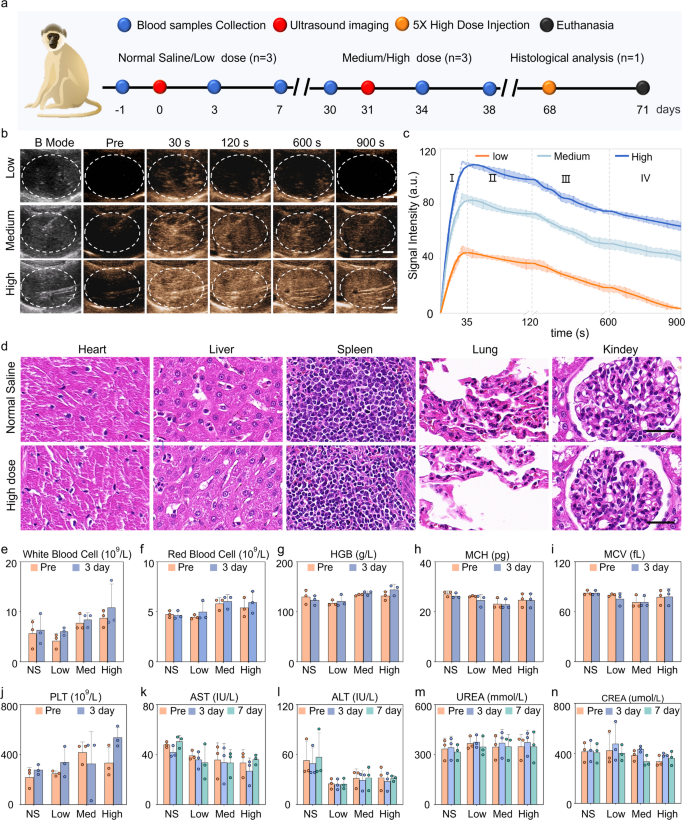
Macaques are extremely just like people by way of tissue construction, physiology, and metabolism. Therefore, we additional investigated the imaging efficiency of PEG-GVs in macaques. The experimental course of is depicted in Fig. 7a. Determine 7b and c demonstrated that every dose of PEG-GVs might yield a speedy enhancement, reaching a peak about 35 s after systemic administration, then step by step decreased over time. With the rise of bubble doses, the distinction sign intensities elevated and persevered for an extended period. Notably, 15-min period was exhibited for medium and excessive dose teams. Quantitative information indicated that the distinction sign intensities of three group at peak achieved 48.44 ± 3.70 a.u, 86.15 ± 3.11 a.u, 113.8 ± 3.66 a.u., respectively. It’s price noting that medium and excessive dose teams remained practically half of the sign intensities after 900 s following the injection of PEG-GVs (Fig. 7c). To confirm the in vivo biosafety of PEG-GVs, histological evaluation of main organs and serum biochemical assays had been performed on the endpoint. HE staining information indicated that there was no important harm to the guts, lung, kidney, and liver within the excessive dose macaque group (Fig. 7d), just like the outcomes obtained in mice (Determine S9). Exams of blood samples, together with liver operate, kidney operate, and blood depend, additional indicated that PEG-GVs didn’t trigger important uncomfortable side effects (Fig. 7e-n, Determine S10). Due to this fact, PEG-GVs, as a novel ultrasound distinction agent, would possibly maintain broad prospects for future scientific functions.
Liver CEUS imaging and biosafety of PEG-GVs in macaque. a, Schematic diagram of biosafety evaluation in macaques. b-c, CEUS photographs of macaque liver (b) and the sign depth curves of the three ultrasonic brokers in 4 phases (c). d, HE staining of main organs of macaques acquired with regular saline or high-dose PEG-GVs. e-n. Hematological detection of every index earlier than and after systemic administration of regular saline or PEG-GVs.